This experiment aimed to extract DNA from cheek cells and use gel electrophoresis to determine the length of unknown DNA fragments. DNA was extracted by shaking Gatorade in the mouth and then mixing it with detergent, pineapple juice, and isopropyl alcohol to precipitate the DNA. The extracted DNA was visualized at the alcohol-solution interface. For gel electrophoresis, a DNA sample containing a dye mixture, unknown DNA, and a DNA marker was loaded onto an agarose gel, and an electric field was applied to separate the fragments by size. The distance traveled by the DNA marker allowed us to estimate the length of the unknown DNA fragment. The results showed that the length of the unknown DNA fragment was approximately 2.7 kilobases (kb) based on a semi-log standard curve. This experiment successfully demonstrated DNA extraction and electrophoresis, providing insight into molecular biology techniques for DNA analysis.
Gel electrophoresis separates DNA, RNA, or proteins by size (Hames et.al, 1990). Each DNA molecule is a double helix composed of two complementary nucleotide strands held together by hydrogen bonds between guanine (G)-cytosine (C) and adenine (A)-thymine (T) base pairs. is. DNA is composed of a negatively charged phosphate backbone, which causes it to migrate towards the positive electrode under an electric field (Alberts et. al., 2002). Dyes such as ethidium bromide and SmartGlow Pre Stain (a non-carcinogenic alternative) visualize DNA by fluorescing under UV light. Molecules move through the pores of the gel at a speed inversely proportional to their length. Smaller fragments move faster (Mika et. al, 2024).
Agarose gels are primarily used in gel electrophoresis, which applies an electric field to separate biomolecules based on size and charge. The gel matrix acts as a molecular sieve, allowing smaller molecules to move faster than larger molecules. Agarose gel is a gel-like substance derived from agar. A polysaccharide obtained from red algae. It is widely used in molecular biology for the separation and analysis of nucleic acids (DNA and RNA) and proteins. Gel concentration affects pore size and resolution, and migration distance can be plotted against the logarithm of molecular length. Using DNA markers or ladders with known fragment sizes allows for accurate size estimation of unknown samples by comparing distance traveled. The length of unknown DNA can be calculated by constructing a standard curve from molecules of known length, typically expressed in kilobases (kb) or base pairs (bp) (Lee et. al, 2012) .
The purpose of this experiment is to measure the length of an unknown DNA fragment by analyzing its electrophoretic migration compared to a standard DNA fragment of known length.
If an unknown DNA fragment migrates similarly to a standard DNA fragment of known length, its size can be estimated based on that comparison.
DNA extraction from cheek cells
DNA extraction from cheek cells involved shaking 5 ml of Gatorade in the mouth for 2 min and then transferring the solution to a test tube. After adding 2 ml of dish detergent and swirling the tube to mix, 2 ml of pineapple juice was added to the solution. Mix by inverting the tube. Next, 2 ml of ice-cold isopropyl alcohol was gently added and the tubes were left for 10 min to precipitate the DNA (Mika et al., 2024).
Preparation of agarose gel
Agarose gels were prepared in advance and stored with a gray plastic comb attached for the next experiment. This comb was carefully removed after the gel solidified and stored for reuse. Each student was assigned a specific gel and electrophoresis unit, and the chamber was designed to accommodate one gel. When loading samples, it was recommended to skip the edge wells to prevent contamination, and students were encouraged to load two samples of each DNA type if desired.
agarose gel electrophoresis
Each gel was loaded with three types of samples: dye mixture, unknown DNA, and DNA marker, and each well contained approximately 25 μL of sample.
DNA samples were prepared and frozen before the experiment. A micropipette with a disposable pipette tip was used to load the samples. By dipping the tip into the sample solution and pressing the thumb button, 25 μL of sample was aspirated into the pipette tip. Care was taken to check and expel any air bubbles before carefully directing the pipette tip into the gel immersion well. Each well was filled to a volume of 25 µL without overfilling, and a new pipette tip was used for each sample to avoid cross-contamination. After filling, the sample vials were returned to the freezer.
Once the DNA sample was loaded, the gel was placed in the electrophoresis chamber with the wells closest to the black (negative) electrode so that the DNA migrated toward the red (positive) electrode. A buffer solution (0.04 M Tris-acetate EDTA, pH 8.0) was prepared in advance and cooled, and approximately 200 mL of this cooled buffer was added to completely cover the gel and remove any trapped air bubbles. I gently placed the orange lid on top and adjusted it as needed.
An 8 cm transparent ruler was placed on the lid and aligned with the well to visually monitor DNA migration. The gel electrophoresis unit was then connected to the power source and the settings were adjusted to run at 100 volts for 35 min. I set the timer, checked all the parameters and pressed the start button. The progression of DNA migration is observed by turning on a blue LED light located at the bottom of the chamber, illuminating the bands as they move away from the well.
Electrophoresis was continued until the dye mixture moved within 2–3 mm of the edge of the gel. After running, the unit was powered off and the cable was disconnected. The gel was visualized under blue LED light and photographed using a black imaging box with an orange filter taped to the lid. I was careful to align the camera and ruler to accurately measure the band.
data analysis
After imaging, a ruler was used to measure the distance traveled by the DNA marker fragment in mm. The results were recorded in a designated table for analysis. This distance was plotted on a semi-log graph against the known size of the DNA marker to estimate the length of the unknown fragment in kilobases (kb). The distance traveled by the unknown DNA fragment was measured from the well to the tip of the band. Using a standard curve, the corresponding value on the x-axis of the graph is the length of the unknown DNA fragment (Mika et al., 2024).
simplified protocol
DNA extraction from cheek cells:
- Hold 5 mL of Gatorade in your mouth for 2 minutes.
- Transfer the solution to a test tube.
- Add 2 mL of dish detergent and stir.
- Add 2 mL of pineapple juice and mix by inverting the tube.
- Slowly add 2 mL of ice-cold isopropyl alcohol.
- Leave the tube for 10 minutes to precipitate the DNA.
Agarose gel electrophoresis:
- Prepare the agarose gel in advance and store it with a gray plastic comb.
- Once the gel has set, carefully remove the comb and reuse it.
- Assign each student a specific gel and electrophoresis unit.
- Skip the endwells to avoid contamination when loading samples.
- Load three samples into the wells (25 μL per well): dye mixture, unknown DNA, and DNA marker.
- Prepare and freeze DNA samples before the experiment.
- Load the sample into the wells using a micropipette with a disposable pipette tip.
- Make sure there are no air bubbles in the pipette tip and use a new tip for each sample.
- Ensure that the gel well in the electrophoresis chamber is placed near the black (negative) electrode.
- Prepare 200 mL of buffer (0.04 M Tris-Acetate EDTA, pH 8.0) in advance and cool it.
- Add chilled buffer to completely cover the gel and remove any air bubbles.
- Place the orange lid on the electrophoresis chamber and adjust as necessary.
- Use an 8 cm transparent ruler to fit the wells and visually monitor the movement of the DNA.
- Set the electrophoresis unit to 100 volts for 35 minutes.
- Observe the DNA transfer using the blue LED light located below the chamber.
- Continue electrophoresis until the dye mixture is 2-3 mm from the edge of the gel.
- Once done, power off the unit and remove the cable.
- Visualize the gel under blue LED light and take a photo using a black imaging box with an orange filter.
- To accurately measure the band, align the camera and ruler.
Data analysis:
- Use a ruler to measure the distance traveled by the DNA marker fragment in mm.
- Record the measurements in a table for analysis.
- Plot the distance traveled against the known DNA marker size on a semi-log graph.
- Use a standard curve to estimate the length of the unknown DNA fragment in kilobases (kb).
result
The DNA extracted from the cheek cells was successfully precipitated and became visible at the alcohol-solution interface in the test tube.
The migration distance of DNA marker fragments was measured. Distances were plotted on a semi-log graph.
| DNA marker fragment number | DNA Manufacturer fragment length (KBP) | DNA Marker fragment movement distance (mm) |
| 1 | 23.13 | 18 |
| 2 | 9.41 | twenty five |
| 3 | 6.68 | 30 |
| 4 | 4.36 | 38 |
| 5 | 2.32 | 58 |
| 6 | 2.03 | 63 |
Table 1: DNA marker fragment length
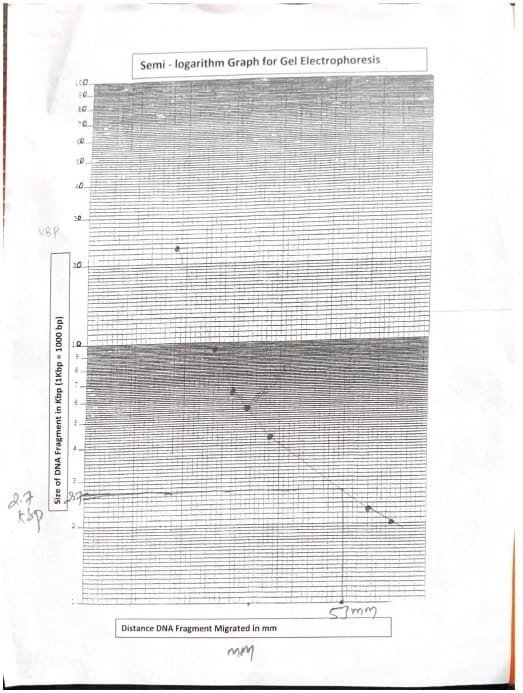
The length of the fragment was determined by measuring the distance traveled by the unknown DNA fragment and comparing it with the corresponding value of the semi-log paper marker.
| Unknown DNA fragments transferred (mm) | unknown DNA fragment length (KBP) |
| 53mm | 2.7KBP |
Table 2: Determination of unknown DNA fragment length
discussion
DNA was extracted from cheek cells by adding different solutions. This facilitated precipitation of the DNA, making it visible at the alcohol-solution interface in the test tube. DNA molecules are too small to be visualized and can only be seen using an electron microscope, but they are made visible through aggregation (Mika et. al., 2024).
Gel electrophoresis experiments utilized agarose gels as a medium and effectively demonstrated the separation of DNA fragments based on size (Hames et. al., 1990). 0.04 M Tris-acetate EDTA buffer at pH 8.0 promoted the migration of negatively charged DNA to the positive electrode (Alberts et. al., 2002). A standard curve constructed from known DNA markers allowed us to estimate the length of the unknown fragment to be approximately 2.7 kilobases (kb).
The clear separation of the dye mixture confirmed the integrity of the electrophoretic process and indicated that the gel was functioning properly. Visualization under blue light allowed the DNA bands to be clearly imaged, and careful alignment with the ruler ensured accurate distance measurements.
Overall, this experiment successfully illustrated the principles of gel electrophoresis and highlighted the importance of clean sample preparation and experimental conditions to obtain reliable results. Future studies may investigate different agarose concentrations or incorporate additional controls to further enhance the analysis.
References
- Hames, B. D. & Rickwood, D. (1990). Gel electrophoresis of nucleic acids: a practical approach. Oxford University Press.
- Alberts B, Johnson A, Lewis J et al. Molecular biology of the cell. 4th edition. New York: Garland Science. 2002. DNA structure and function. Available from: https://www.ncbi.nlm.nih.gov/books/NBK26821/
- Mika, TA, Klein, RJ, Brajahn, AE, Connor, RL, Swimmer, LM, White, R.
- E., Gosses, M. W., Carter, T. E., Andrews, A. M., Maier, J. L., & Sidiq, F. (Eds.). (2024). Anatomy and Physiology BIO 211 Laboratory Manual (3rd edition). Owens Community College.
- Lee, P. Y., Costumbrado, J., Hsu, C. Y., and Kim, Y. H. (2012). Agarose gel electrophoresis for separation of DNA fragments. Visualization Experiment Journal: JoVE(62), 3923. https://doi.org/10.3791/3923







