Cell biology delves into the complex ways cells maintain their internal environment, interact with their surroundings, and regulate their functions. Integral to these processes are passive and active transport mechanisms. These systems control the movement of vital substances in and out of cells. In this article, we will discuss these mechanisms, focusing on the role of membrane proteins in tonicity, diffusion, osmolarity, and active transport.
Osmotic pressure and its effects on cells
Tonicity describes how the concentration of solutes in a solution affects the movement of water through the cell membrane. Understanding tonicity is important for understanding how cells maintain their shape and function in different environments.
Hypotonic solution
A hypotonic solution has a lower concentration of solutes than inside the cell, resulting in a higher concentration of water molecules outside the cell. This imbalance has several effects on the cell:
- Osmotic gradient: Water moves into cells due to an osmotic gradient caused by a low solute concentration outside the cell. Osmosis is the movement of water through a semipermeable membrane from an area of low solute concentration to an area of high solute concentration. The cell membrane allows the passage of water but restricts the movement of solutes.
- Cell expansionWhen water enters a cell, it accumulates in the cytoplasm, causing the cell to swell. This swelling increases internal pressure and can stretch the cell membrane.
- Melting risk: Sustained hypotonicity leads to the ingress of excess water, increasing intracellular pressure and potentially causing cells to rupture or lyse, releasing their contents into the extracellular space.
Isotonic Solution
An isotonic solution matches the solute concentration in the cytoplasm of a cell. The main characteristics of an isotonic solution are:
- Balanced OsmolalityIn this environment, the osmotic pressures inside and outside the cell are equal, so the movement of water in and out of the cell is balanced and the amount of water does not change.
- Stable Cell VolumeA balanced transfer of fluid allows cells to maintain their normal shape and volume. Isotonic solutions are essential in medical procedures such as intravenous fluid infusions to prevent cells from swelling and shrinking.
Hypertonic solution
A hypertonic solution contains a higher concentration of solutes than the inside of a cell. Effects on cells include:
- Osmotic gradient: Because of the high concentration of solutes outside the cell, water is pumped out of the cell into the hypertonic ECF. This movement equalizes the solute concentration across the membrane.
- Cell shrinkageLoss of water causes cells to shrink, a process called crown formation. This shrinkage impairs cell function and, if severe or prolonged, can lead to cell damage.
- Medical UseHypertonic solutions can be used therapeutically to treat conditions such as edema by drawing excess water out of tissues.
Osmosis and diffusion are the basic passive transport mechanisms that involve different substances and processes.
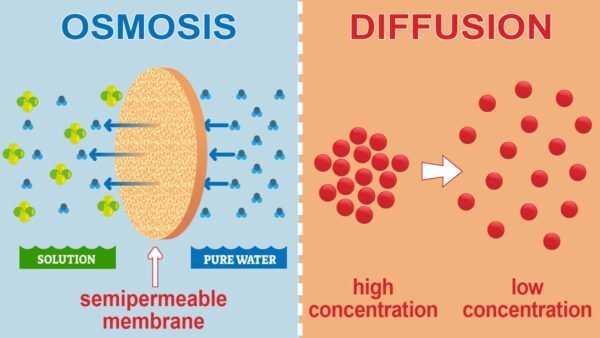
diffusion
- meaningThe process of diffusion is the movement of a solute from an area of higher concentration to an area of lower concentration. This process continues until the concentration of the solute reaches equilibrium.
- processDiffusion: Solute particles can pass freely through cell membranes if they are permeable. This mechanism does not require energy (ATP). For example, the spread of perfume in a room is evidence of diffusion.
Penetration
- meaningOsmosis is a type of diffusion that focuses on the movement of water across a semipermeable membrane from an area of lower solute concentration (hypotonic) to an area of higher solute concentration (hypertonic).
- processOsmotic pressure balances the concentration of water on either side of the membrane. In an isotonic solution, water movement is balanced. However, in hypotonic and hypertonic solutions, water adjusts to balance the concentration of solutes.
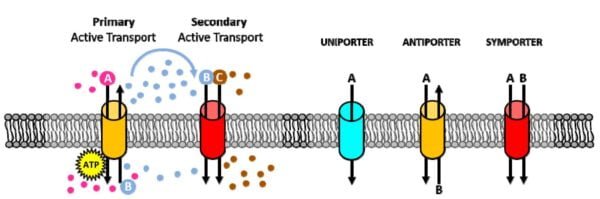
Active transport through membrane proteins
Active transport is essential for moving substances across cell membranes against a concentration gradient. Unlike passive transport, which relies on a natural gradient, active transport requires energy in the form of adenosine triphosphate (ATP).
Primary active transport
- Sodium-potassium pumpThis pump is essential for maintaining a gradient of sodium and potassium ions across the cell membrane. For each ATP molecule hydrolyzed, it transports three sodium ions out of the cell and two potassium ions into it. This process creates a gradient that is essential for many cellular functions, including the transmission of nerve impulses and muscle contraction.
Secondary active transport
- mechanismSecondary active transport utilizes ATP indirectly, by taking advantage of the gradient established by primary active transport. For example, the sodium gradient generated by the sodium-potassium pump helps to transport substances such as glucose against their gradient.
- Joint transportThis mechanism often involves symporters that use the movement of sodium ions up a gradient to move other substances against the gradient.
Types of Membrane Pumps
- Uniport Pump: They transport a single substance in one direction across a membrane. For example, calcium pumps help maintain low calcium levels inside cells by moving calcium ions out of the cell.
- Symport Pump: These move two or more substances in the same direction. An example is the sodium-glucose cotransporter, which transports both sodium ions and glucose into the cell at the same time.
- Antiport PumpThey transport substances in opposite directions. The sodium-potassium pump is a classic example of countertransport, transporting sodium ions out of the cell and potassium ions into the cell.
Active transport by vesicles
When a substance is too large or too polar to pass directly through the cell membrane, cells use vesicular transport.
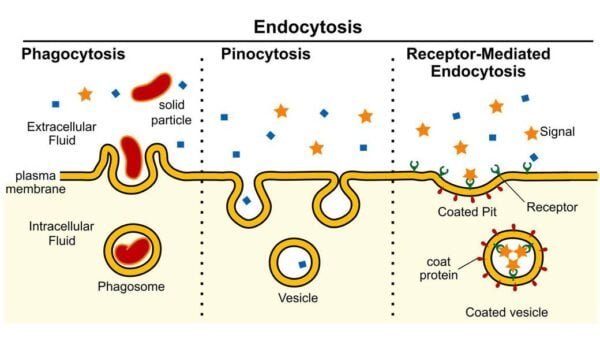
Vesicle transport
- Vesicle: Small membrane-bound sacs that transport larger molecules and particles in and out of cells. Vesicles can fuse with cell membranes to release their contents or form from membranes to ingest materials.
- Energy requirementsVesicle transport is an active process that requires ATP to move vesicles and their contents across the cell membrane.
Endocytosis
- PhagocytosisIn this process, often referred to as “cell eating,” large particles such as bacteria or dead cells are engulfed. The engulfed material is trapped in the phagosome, which then fuses with the lysosome for digestion.
- PinocytosisIn this process, known as “cell drinking,” cells ingest droplets of liquid from the extracellular space. Tiny vesicles encapsulate the liquid and process it inside the cell.
- Receptor-Mediated EndocytosisThis particular type of pinocytosis uses surface receptors to selectively internalize certain molecules, such as hormones or cholesterol, whose binding to the receptors triggers vesicle formation and internalization.
Exocytosis
- processExocytosis is the opposite of endocytosis. It occurs when vesicles fuse with the cell membrane to release their contents outside the cell. This process is essential for the secretion of hormones, neurotransmitters, and other essential molecules.
Conclusion
A comprehensive understanding of the mechanisms of passive and active transport is fundamental to cell biology. Passive processes, such as diffusion and osmosis, depend on a concentration gradient and do not require energy. Conversely, active transport processes, such as primary and secondary transport, utilize energy to move substances against a gradient. In addition, vesicular transport can transport molecules that are too large to pass directly through the cell membrane.
These transport mechanisms are essential for maintaining cellular homeostasis, facilitating communication between cells and allowing cells to adapt to their environment. Mastering these concepts will provide valuable insight into the complex and dynamic nature of cellular life.